Abstract
The addition of Gemtuzumab Ozogamicin (GO) to induction chemotherapy has been demonstrated to improve outcomes in older adults with non-adverse cytogenetic risk acute myeloid leukemia (AML) but it is currently uncertain whether a higher dose, delivered as a fractionated schedule as per the ALFA0701 trial, provides a survival benefit over a single lower dose (GO 3mg/m2) as given in the NCRI AML16 trial. The AML18 trial evaluated this and we now report 5yr overall survival (OS) results together with information from response assessment by measurable residual disease (MRD) and molecular stratification.
Methods The NCRI AML18 trial randomised older AML or high-risk MDS patients (>10% blasts) who were not known to have adverse cytogenetics at trial entry to receive course 1 DA induction (Daunorubicin 60mg/m2 d1, 3, 5, AraC 100mg/m2 bd d1-10) with either GO 3mg/m2 on day 1 (GO1), or fractionated GO on days 1 and 4, maximum 5mg per dose (GO2). Further courses did not include GO. Of 852 enrolled patients, 8 withdrew trial consent. Baseline cytogenetics and molecular profiling included a 95-gene targeted NGS panel. Following course 1 MRD response was assessed by flow cytometry (results available for 608 patients including 453 in remission [CR+CRi] post course 1) with any measurable level of MRD considered positive. Randomisations for course 2 were MRD directed and will be reported separately.
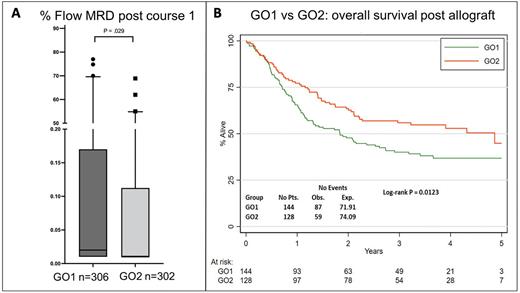
Results Treatment arms (GO1, n=422; GO2, n=422) were balanced for baseline characteristics. Median age was 68 years. 79.7% had de novo AML, 10.6% clinical secondary AML, 9.7% high risk MDS. Of patients with cytogenetic data (n=782), 4.4% and 80.9% had favourable or intermediate risk cytogenetics respectively. 114 patients (14.6%) were found to have adverse risk cytogenetics after trial entry. Median follow-up was 50.2 months (quartiles: 44 to 60). GO1 and GO2 were comparable for day 30 mortality (7.6% vs 8.0%), time to count recovery (median 29 vs 29 days for neutrophils to 1 x 109, median 29 vs 30 days for platelets to 100 x 109/L), toxicity profiles and post course 1 response rates (56.6% vs 62.5% for CR, 63.5% vs 67.3% for CR+CRi). However lower post course 1 MRD levels (Figure A) and a higher MRD negative response rate were observed for the GO2 arm (57.3% GO2 vs 49.7% GO1 for MRD negative, 72.2% GO2 vs 62.4% GO1 for MRD <0.1%). This differential response for GO2 versus GO1 varied across molecular subtypes, greatest for IDH mutations (MRD <0.1%, 71% GO2 vs 47% GO1 for IDH2, 76% GO2 vs 62% GO1 for IDH1) and then NPM1 mutated patients (MRD <0.1%, 81% GO2 vs 73% GO1).
5yr OS rates were 29% for GO2 and 24% for GO1 patients (p=0.14). In a sensitivity analysis excluding patients with adverse cytogenetics / TP53 mutations, 5yr OS rates were 33% for GO2 and 26% for GO1 (p=0.045). Survival advantage from GO2 vs GO1 was most apparent for the subgroup of patients with DNA methylation-type mutations (including mutated IDH 1/2, DNMT3A) (HR 0.77, 95%CI 0.63-0.93). 27% of patients received a CR1 allogenic stem cell transplant (ASCT) (122 GO1, 106 GO2) with a survival advantage for transplant on Mantel-Byar analysis (HR 0.76, 95%CI 0.62-0.93, P=0.007). Survival was superior for transplanted patients on the GO2 arm compared to GO1 (HR 0.66, 95%CI 0.47-0.91, P=0.01) (Figure B). The overall survival benefit from GO2 was lost when patients were censored at ASCT. Additionally, GO2 did not provide a survival advantage for patients over 70yrs by age subgroup analyses (test for trend, P=0.019).
Conclusions Compared to single dose GO, the fractionated schedule (GO2) was associated with greater reduction in MRD, and improved overall survival in older adults with non-adverse risk genetics; comparable differences by molecular subtype were observed for MRD response and survival. AML18 trial results suggest that the differential benefit from GO2 for older adults is dependent on delivery of ASCT in order to translate the better leukemia clearance from induction into a significantly higher 5yr survival.
Disclosures
Freeman:Neogenomic: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Research Funding; JAZZ: Research Funding, Speakers Bureau. Vyas:Celgene: Honoraria, Research Funding; Pfizer: Honoraria; Abbvie: Honoraria; Daiichi Sankyo: Honoraria; JAZZ: Honoraria; Bristol Myers Squibb: Research Funding; Astellas: Honoraria. Mehta:Astellas: Honoraria, Speakers Bureau; Abbvie: Honoraria, Speakers Bureau; Jazz: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau. Knapper:Novartis: Research Funding, Speakers Bureau; Servier: Consultancy, Honoraria; BMS: Consultancy; Astellas: Speakers Bureau; Jazz: Consultancy, Honoraria, Speakers Bureau. Russell:Astellas: Honoraria; Servier: Honoraria; Jazz Pharma: Research Funding; Pfizer: Honoraria, Research Funding, Speakers Bureau.
OffLabel Disclosure:
Gemtuzumab Ozogamicin used as single dose
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal